Oxidation Number of Hydrogen
1 In hydrogen peroxide each hydrogen still has an oxidation number of 1 because each hydrogen gives up a single electron to oxygen. The boron atom has 3 valence electrons.
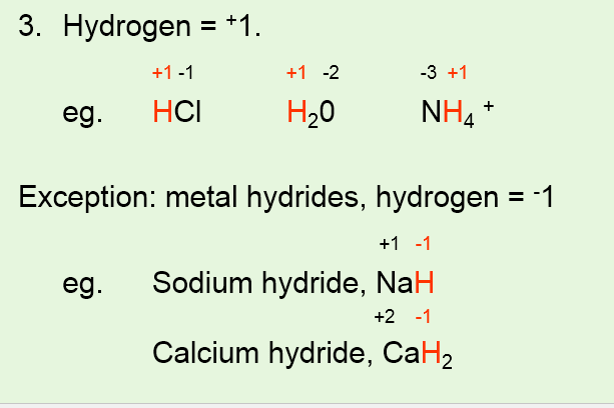
Oxidation Numbers Vce Chemistry
For the compound hydrochloric.
. The atoms in He and N 2 for example have oxidation numbers of 0. For example in LiH and NaH its oxidation. The oxidation number of hydrogen is 1 when it is combined with a nonmetal as in CH 4 NH 3 H 2 O and HCl.
Because the single oxygen atom has received a total of two electrons one from each hydrogen oxygen has. When H is directly attached to strongly electropositive metals such as K the H atom is present in the form. Hydrogen has an oxidation number of 1 when combined with non-metals but it has an oxidation number of -1 when combined with metals.
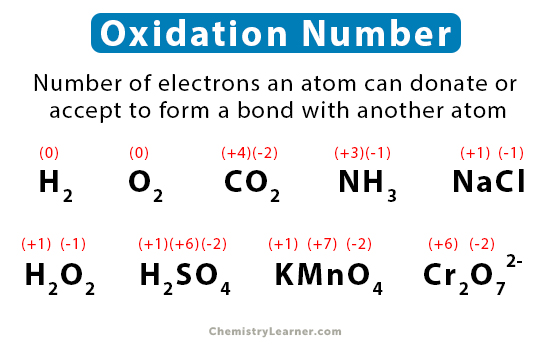
The oxidation number of a free element is always 0. Hydrogen usually an oxidation number of 1 when it combines with a non-metal and an oxidation number of -1 when it combines with a metal. For boron that is 204 and for hydrogen it is a little bit larger 220 Dynamic Periodic Table This means that H is the.
Here two atoms of hydrogen are bonded together as such. Hydrogen has an oxidation number of 1 in water because it has lost one electron. If the hydrogen is part of a binary metal hydride.
What is the oxidation number of hydrogen in h2o2. Li Ca2 and Al3 have oxidation states of 1 2 and 3 respectively. The oxidation number of a monatomic ion equals the.
In a neutral compound all Oxidation Numbers must add up to zero. The oxidation state of hydrogen in a compound is usually 1. The algebraic sum of the oxidation numbers of.
Monoatomic ions have an oxidation number equal to their charge. It can form covalent bond with three hydrogens to form BH3 but it is still short of two electrons to achieve octet number of electrons. Oxidation number of hydrogen in proton H is 1 and in hydride is -1.
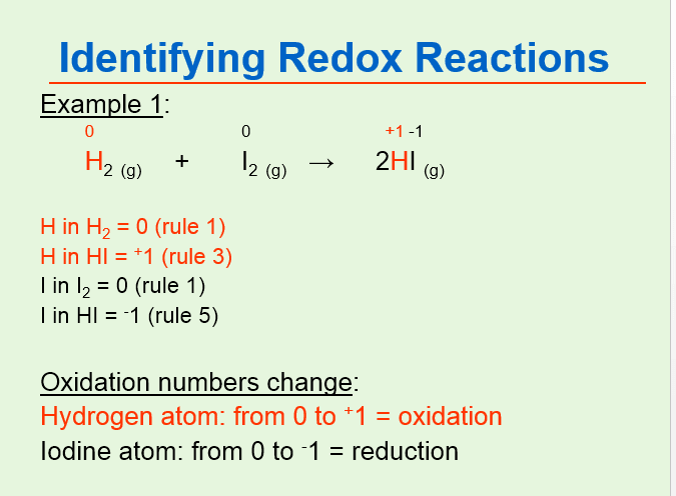
In this molecule the hydrogen exhibits oxidation number zero 0 since when two or more atoms of a type combine. The sum of oxidation states of all atoms in a neutral compound is zero while in a charged species it is equal to the net. To answer that you need to look at the electronegativity.
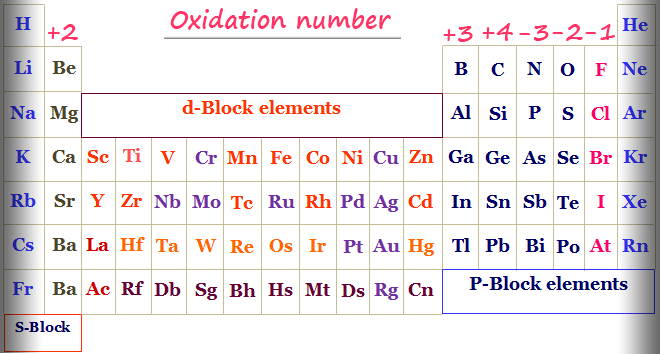
Group 1 1 Group 2 2 Hydrogen with Non-Metals 1 Hydrogen with Metals or Boron -1 Fluorine. Yes in the elementary hydrogen when it assumes the diatomic molecule H2. Oxidation number of hydrogen rm 1 Oxidation number of oxygen is rm 2.
In KH the oxidation number of hydrogen is -1 and the oxidation number of K is 1. 6 The oxidation number of hydrogen is 1 when it is combined with more electronegative elements most nonmetals and 1 when it is combined with more electropositive elements. Answer 1 of 2.
The oxidation number of hydrogen is 1 except when it is bonded to metals in binary compounds that is compounds containing two elements. The general oxidation state of oxygen is 2 but in peroxides it is 1. The oxidation number of hydrogen is -1 when it is combined with a metal as in.
H 2 is a free element meaning it is not bound to any atoms other than its own. If the oxygen is bonded to fluorine the number is 1. It does not have an overall charge and is a neutral.
Cl- and SO42- have oxidation states. People also asked Study. Oxidation number of oxygen in oxide ion O 2- is -2 and in peroxide ion O-O 2- is -1.
Hydrogen has an oxidation number of 1 when it is bonded to nonmetals which are highlighted on the right side of the following periodic table. In borane even though B is a non-metal each hydrogen displays an oxidation state of -1 again electronegativity of H is 21 as opposed 20 of B Hope this makes sense.
Oxidation Number State Definition Rules How To Find And Examples
Oxidation Number Periodic Table Elements Definition Rules

Oxidation Numbers Vce Chemistry
Rules For Oxidation Numbers Study Guide Inspirit
0 Response to "Oxidation Number of Hydrogen"
Post a Comment